The search for a stem cell treatment has long been the big hope – a kind of “holy grail” –for spinal cord injury research projects. It just makes sense–to medical researchers and neuroscientists as well the rest of us–that stem cell treatments ought to work.
Here’s why: Stem cells have the amazing potential to renew themselves, and to develop into many different types of cells in the body. So injection of stem cells should logically be able to reverse or repair damage to the spinal cord and restore some feeling and movement after an injury.
However, the “miracle” cures we often read about somehow never pan out. The research turns out to have major flaws; the “results” don’t last or can’t be duplicated. Sometimes, the “study” itself is actually a scam to lure hopeful people with spinal cord injuries into undergoing expensive treatments in a foreign country. We don’t usually report such studies on our website or in this newsletter for fear of raising false hopes.
However, recently published preliminary reports of new stem-cell research from two highly-reputable sources – Yale University and the Mayo Clinic — show promise that we want you to know about. Both use mesenchymal stem cells (MSCs) taken from the patients’ own bodies, and both have improved feeling and movement in people with spinal cord injuries.
Here’s more information on stem cells and the new research:
Video: Stem cells: A step toward improving function after spinal cord injury
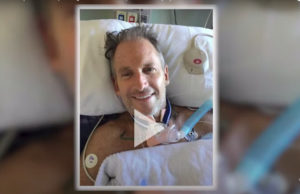 Chris Barr is the first patient in the Mayo Clinic stem cell clinical trial. His story is exciting, heart-warming and encouraging. “The recovery that he had was beyond our expectations,” says Dr. Mohammed Bydon. “Keep in mind that not every patient will have the same benefit.”
Chris Barr is the first patient in the Mayo Clinic stem cell clinical trial. His story is exciting, heart-warming and encouraging. “The recovery that he had was beyond our expectations,” says Dr. Mohammed Bydon. “Keep in mind that not every patient will have the same benefit.”
Mayo Clinic research is a step toward hope for spinal cord injuries
By Susan Buckles
Early research published in Mayo Clinic Proceedings examines the first case at Mayo Clinic of stem cell therapy tested in humans for spinal cord injury. The case study found stem cell intervention, which took place after standard surgery, and physical and occupational therapy, restored some function in a patient with spinal cord injury. The report, “Celltop Clinical Trial: First Report From a Phase I Trial of Autologous Adipose-Derived Mesenchymal Stem Cells in the Treatment of Paralysis Due to Traumatic Spinal Cord Injury” is published in the Nov. 27, 2019 edition of Mayo Clinic Proceedings.
The research discusses the experience related to the first case in a phase I safety study of mesenchymal stem cell treatment for spinal cord injury. Mohamad Bydon, M.D., a Mayo Clinic neurologic surgeon and the lead author, cautions that each patient is different, so it’s too early to consider stem cell therapies as a treatment or cure for paralysis from spinal cord injury. Dr. Bydon adds that much like early trials in general, the stem cell trials are going to show variable response rates.
“While in this case, the first subject was a superresponder, others may not respond in the same manner. We do not yet understand all of the necessary biology needed to achieve neurological recovery in paralyzed individuals,” says Dr. Bydon. “One of our objectives in this study and future studies is to better delineate who will be a responder and why patients respond differently.”
The research
The research centers on a 53-year-old man who suffered a spinal cord injury in a surfing accident that left him paralyzed below the neck. The patient had immediate improvements with standard therapy, but plateaued at six months post-injury. Researchers enrolled the patient in the study at Mayo Clinic nine months after the accident and injected the patient with stem cells 11 months after injury. After the stem cell injection, the patient significantly improved motor and sensory function.
The case study focuses on feasibility, safety and dosing of stem cell therapy. The study team derived mesenchymal stem cells from the patient’s fat cells and injected them into the lower back in a procedure known as lumbar puncture.
Dr. Bydon; Wenchun Qu, M.D., Ph.D., a physical medicine and rehabilitation physician; and Allan Dietz, Ph.D., a transfusion medicine physician, led the multidisciplinary research team at Mayo Clinic.
“Severe spinal cord injury is a devastating condition for which scientists and physicians are trying to find a cure. For the first time, we are inspiring hope that people may receive better recovery in their function and quality of life,” says Dr. Qu. “Mayo Clinic has been taking the lead in translating the fruits of decades of research and treating neurological conditions, among which have been very important clinical trials where we evaluate the safety, feasibility and efficacy of adult stem cells for severe spinal cord injuries.”
“This work both demonstrates the ability of cells to initiate repair and capitalizes on more than 10 years of work in the Immune, Progenitor and Cell Therapeutics Lab at Mayo Clinic. While there is still much to learn about the amazing ability of cells to heal tissue, this trial is an important step in advancing cell-based therapies toward clinical practice,” says Dr. Dietz.
Investigators collected cerebrospinal fluid to look for new biological markers that might give clues to healing. Biological markers are important because they can help identify the critical processes that lead to spinal cord injury at a cellular level and could lead to new regenerative therapies.
Further study is needed to understand the effectiveness of stem cell lumbar injections and why patients may respond differently.
Currently, there is no way to reverse the devastating life-changing effects of paralysis from spinal cord injuries. Today, the only treatment is supportive care, such as surgery and physical and occupational therapy.
Dr. Bydon says his early findings give hope that new regenerative therapies are on the horizon for spinal cord injuries.
“The hope is that we will have novel treatments for spinal cord injuries in the coming years that will be different from what we have today. These will be therapies that do not rely upon supportive care, but therapies that rely on science to create a regenerative process for the spinal cord,” says Dr. Bydon.
This research was made possible by funding from Mayo Clinic Transform the Practice Initiative and Regenerative Medicine Minnesota with support from the Mayo Clinic Center for Regenerative Medicine and the Department of Laboratory Medicine and Pathology Immune, Progenitor and Cell Therapeutics lab. The Transform the Practice Initiative aims to foster multidisciplinary teams of clinicians and researchers who align discovery and translational science, create new capacities and achieve solutions that improve the practice and address the unmet needs of patients.
Yale scientists repair injured spinal cords using patients’ own stem cells
By Lakshmi Bangalore
February 22, 2021
Intravenous injection of bone marrow derived stem cells (MSCs) in patients with spinal cord injuries led to significant improvement in motor functions, researchers from Yale University and Japan report Feb. 18 in the Journal of Clinical Neurology and Neurosurgery.
For more than half of the patients, substantial improvements in key functions — such as ability to walk, or to use their hands — were observed within weeks of stem cell injection, the researchers report. No substantial side effects were reported.
The patients had sustained non-penetrating spinal cord injuries, in many cases from falls or minor trauma, several weeks prior to implantation of the stem cells. Their symptoms involved loss of motor function and coordination, sensory loss, as well as bowel and bladder dysfunction. The stem cells were prepared from the patients’ own bone marrow, via a culture protocol that took a few weeks in a specialized cell processing center. The cells were injected intravenously in this series, with each patient serving as their own control. Results were not blinded and there were no placebo controls.
Yale scientists Jeffery D. Kocsis, professor of neurology and neuroscience, and Stephen G. Waxman, professor of neurology, neuroscience and pharmacology, were senior authors of the study, which was carried out with investigators at Sapporo Medical University in Japan. Key investigators of the Sapporo team, Osamu Honmou and Masanori Sasaki, both hold adjunct professor positions in neurology at Yale.
Kocsis and Waxman stress that additional studies will be needed to confirm the results of this preliminary, unblinded trial. They also stress that this could take years. Despite the challenges, they remain optimistic.
“Similar results with stem cells in patients with stroke increases our confidence that this approach may be clinically useful,” noted Kocsis. “This clinical study is the culmination of extensive preclinical laboratory work using MSCs between Yale and Sapporo colleagues over many years.”
“The idea that we may be able to restore function after injury to the brain and spinal cord using the patient’s own stem cells has intrigued us for years,” Waxman said. “Now we have a hint, in humans, that it may be possible.”
This primer on stem cells is intended for anyone who wishes to learn more about the biological properties of stem cells, the important questions about stem cells that are the focus of scientific research, and the potential use of stem cells in research and in treating disease.
1. Introduction: What are stem cells, and why are they important?
2. What are the unique properties of all stem cells?
3. How do you culture stem cells in the laboratory?
4. How are stem cells used in biomedical research and therapies?
5. How does NIH support stem cell research?
This is an important resource that you can trust. It includes detailed information on clinical trials.

Greetings!
Nice blog and thank you for sharing it.
Can you help me get regenerative medicine
Please
I need to go back to work