Editor’s Note: It’s a common theoretical question: “Suppose they suddenly found a cure for your disability? — What would you do?” Our special contributor, Ben Mattlin, is currently grappling with that very question—and it’s not theoretical.
I was born before my disability could be diagnosed. Now I’m told it can be cured.
 I’m not a science guy, but I do like solving mysteries. My disability was a mystery for many years. I was born in the early 1960s, when few people (if any) had heard of spinal muscular atrophy (SMA). I’m not even sure the term existed yet, though I understand that a related congenital neuromuscular weakness was first recognized in the 1890s.
I’m not a science guy, but I do like solving mysteries. My disability was a mystery for many years. I was born in the early 1960s, when few people (if any) had heard of spinal muscular atrophy (SMA). I’m not even sure the term existed yet, though I understand that a related congenital neuromuscular weakness was first recognized in the 1890s.
Little Understood for 100 Years
Still, SMA remained little understood and difficult to identify for most of the next century. In the go-go 60s, I was officially dubbed a “floppy baby”—i.e., I couldn’t sit unassisted. I crawled some, but never stood or walked. My weak muscle tone was also described as “amyotonia.”
But in the 1990s, a Dr. Judith Melki and her team found a genetic marker for SMA, which made diagnosing much easier. A simple blood test can show if you carry a gene called survival motor neuron 1 (SMN1). When it’s missing or compromised, your body doesn’t produce the SMN protein that enables muscle-building messages to transmit to and from the brain. This is the gene component I lack.
The good news is, there is also a backup gene called SMN2. It can provide some extra SMN protein, and I have three copies of it. So the extent and/or speed of my muscle deterioration is somewhat mitigated.
Confused? Scientists were too, until recently. It’s a rare condition, I was always told, affecting one in every 6,000 to 10,000 people worldwide. But it’s now thought that one in 40 is a carrier without showing symptoms. If two carriers mate, their offspring have a 50-50 chance of developing SMA.
Two New Treatments
There is not much money in treating rare diseases, and for most of my life there was absolutely nothing for SMA. You could help ease symptoms, such as by using ventilators and nebulizers to ease breathing difficulties (or, for that matter, wheelchairs to enable mobility). These methods have prolonged many lives. But SMA itself was unstoppable.
Then, in late 2016, the FDA approved Spinraza. Developed by Biogen and Ionis Pharmaceuticals, this amazing new drug was purported to be stopping muscle atrophy and saving babies’ lives! People my age were ecstatic as well, because even if it didn’t give them back any muscle tone (you can’t revive dead nerves cells), Spinraza was touted to stop further progression.
Forgive me, but I was not impressed.
I had serious misgivings about the new drug’s promise. Not that I questioned the science, which I didn’t fully understand anyway. But given the risk of possible side effects—principally, kidney toxicity—and the fact that drug approval had come in less than three months under “priority review,” raising doubts about long-term complications; and given my basic comfort level with my disability—after 50-some years, I’m pretty used to it—and the likely possibility that it wouldn’t do me much good at this point anyway, I wasn’t particularly interested.
It was also a major commitment. It has to be injected into the spine several times a year for the rest of your life. Though deals were quickly ironed out between the pharmaceutical manufacturers and insurance providers, the first year can cost $750,000, then about $375,000 annually thereafter.
Zolgensma
I took some flak for my views, published in The New York Times. Many friends and allies were using Spinraza and loving it. Then, not long ago, a new scientific breakthrough caused me to rethink my anti-cure mindset.
The Wall Street Journal was the first I saw to report on a new gene therapy called Zolgensma. Made by AveXis, a Novartis company, it’s touted as having “potential to cure spinal muscular atrophy.” Cure, not treat. That got my heart to go pitter-pat. I knew it was probably hype, but still. This needed further investigating.
How is Zolgensma different from Spinraza? Mayer Winkler, writing in Seeking Alpha, explained the distinction this way: “Will it be the ‘Rent-a-Gene’ model of Spinraza that manipulates existing genetic code but which requires lifetime maintenance, or will it be the ‘Buy-a-Gene’ model of Zolgensma that places new genetic code into patients and fixes the problem with one treatment?”
A One-and-Done Fix?
The appeal of a one-and-done fix is what grabbed me the most. Zolgensma apparently injects the genetic code for functional SMN1 directly into the malfunctioning motor neurons. This makes them start producing the SMN protein by themselves, which is what we SMAers lack. Problem solved.
Spinraza, on the other hand, enables the backup gene SMN2 to produce a little more extra SMN for a few months. It doesn’t even try to fix the broken or missing SMN1; rather, it’s a workaround, a way to bypass the deficiency. That’s why recurring doses are necessary. This genetic detour only works for so long before petering out.
So far, there isn’t enough clinical data on either one to make a totally fair comparison. As of this writing, Zolgensma has only been approved for kids age two and under. Spinraza is approved for just about everybody with SMA.
Just For Kids?
A while back, I asked my neurologist if I was correct in assuming that Spinraza would not give me strength I never had. He confirmed it. It might prevent further muscle loss, or it might do me no good whatsoever. The same is probably true for Zolgensma; in fact, the press release plainly states it is “not a cure and cannot reverse any damage already caused by SMA before treatment.” But it’s certainly easier to take. For all but the smallest babies, it has to be injected into the spine, as does Spinraza, but at least it’s just one time. Even if it didn’t do me any good, it’s less of an investment.
Let me qualify that. This single dose is priced at more than $2 million. It was quickly dubbed the most expensive medicine ever. But over the long run, that’s still cheaper than Spinraza.
Zolgensma does carry warnings of possible liver damage, though that’s supposed to be remedied by low-dose steroids.
What about my abhorrence to the cure mentality—my feeling that it’s tantamount to a betrayal of disability pride? If I’m to love myself as I am, I figured, then I don’t need or want to be fixed, to be made like other people. I believe in valuing my life as it is. I’m not waiting for medicine to make me complete.
Yet I use all kinds of medicines to keep me alive and thriving. Wouldn’t it be foolish to turn down something that could keep me going longer?
Staying Open-Minded
I have to be honest. Even in my current decrepit state, I could grow weaker. I can’t feed myself, I drive my wheelchair with an ultrasensitive lip-controlled mini joystick, and I need hands-on assistance throughout the day. Nevertheless, it could become harder to breathe or swallow or talk, for instance. That would suck. I don’t want or expect to start running marathons, but I’d like to be able to keep chattering and chomping Cheetos. Disability is a huge part of my identity. It’s part of my profession. I don’t know who I’d be if I lost it. But if a one-time magic injection can help me maintain my current status, why not try it?
For now, I’ll wait and see. If Zolgensma is approved for people my age, and it’s shown to be effective with manageable side effects, I’m open to considering it. If that doesn’t happen, I won’t be too disappointed either. After all, I’m pretty satisfied with my life as it is.
###
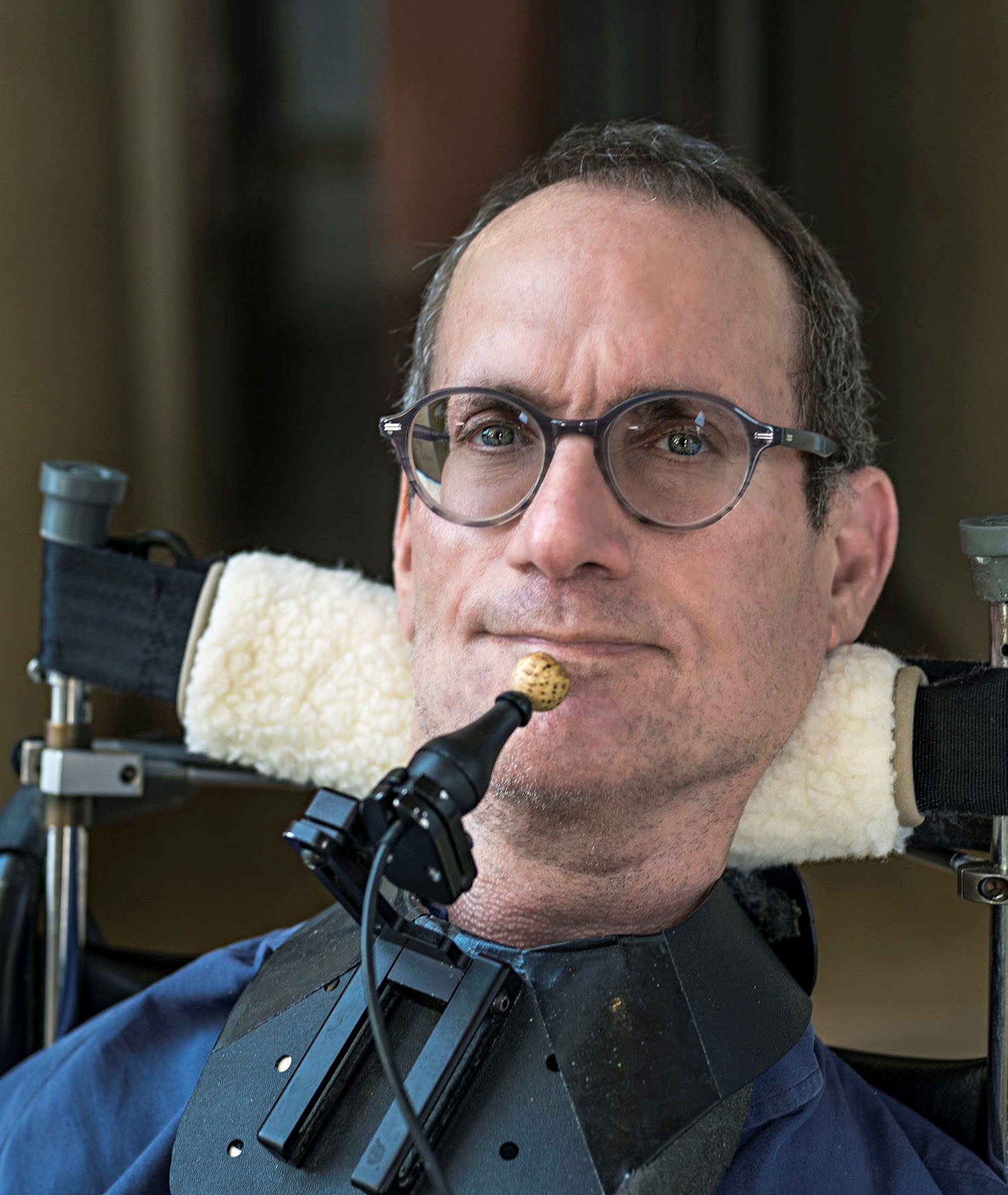 Our special contributor Ben Mattlin, was born with spinal muscular atrophy (SMA), a congenital muscle weakness that causes paralysis and related health issues. A highly regarded writer, Ben’s work has appeared in “The New York Times,” “The Washington Post” and “USA Today.” He lives in Los Angeles with his wife and children.
Our special contributor Ben Mattlin, was born with spinal muscular atrophy (SMA), a congenital muscle weakness that causes paralysis and related health issues. A highly regarded writer, Ben’s work has appeared in “The New York Times,” “The Washington Post” and “USA Today.” He lives in Los Angeles with his wife and children.
Such an awesome article, Mr. Mattlin! I’m so excited for you and look forward to hearing more from you soon!